Introducing Energy Worksheet #1 Reaction Rates, a comprehensive guide designed to illuminate the intricate world of chemical reactions and their rates. This worksheet delves into the factors that govern reaction rates, their significance in various scientific disciplines, and provides a detailed experimental setup to explore these concepts firsthand.
Through a series of engaging activities and thought-provoking questions, this worksheet unravels the complexities of reaction rates, empowering students with a deeper understanding of this fundamental chemical phenomenon.
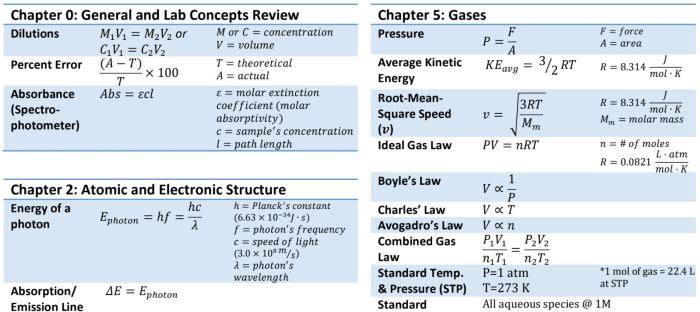
Introduction
In chemical reactions, the reaction rate measures how quickly the reactants are converted into products. Understanding reaction rates is crucial in various fields, including chemical engineering, environmental science, and medicine.
Several factors influence reaction rates, including:
- Concentration of reactants
- Temperature
- Surface area of reactants
- Presence of a catalyst
Experiment Design
The experiment will be conducted in a laboratory setting, and the setup will involve two beakers containing equal volumes of hydrochloric acid (HCl) and sodium hydroxide (NaOH) solutions. A thermometer will be placed in each beaker to measure the temperature change.
The independent variable in this experiment is the concentration of the HCl solution. The dependent variable is the temperature change observed in the beakers. The temperature change will be measured using a thermometer.
Safety Precautions
The following safety precautions should be taken during the experiment:
- Wear gloves and a lab coat when handling chemicals.
- Handle the chemicals with care and avoid contact with skin or eyes.
- Do not mix the chemicals directly; instead, add the acid to the base slowly while stirring.
- Dispose of the chemicals properly according to the laboratory’s safety guidelines.
Data Collection and Analysis
In this section, we will discuss the methods for collecting and analyzing data in the reaction rate experiment. This includes creating an experimental data table, calculating the reaction rate, and discussing statistical methods for data analysis.
Experimental Data Table
To organize the experimental data, we will create a table with the following columns:
- Time (s)
- Concentration of Reactant A (M)
- Concentration of Reactant B (M)
- Reaction Rate (M/s)
Calculating the Reaction Rate
The reaction rate can be calculated using the following formula:
Reaction Rate =
- Δ[A]/Δt =
- Δ[B]/Δt
where:
- Δ[A] is the change in concentration of reactant A over time
- Δ[B] is the change in concentration of reactant B over time
- Δt is the change in time
Statistical Methods for Data Analysis
Once the data has been collected, it can be analyzed using statistical methods to determine the significance of the results. Some common statistical methods used in reaction rate analysis include:
- Linear regression
- Correlation analysis
- Analysis of variance (ANOVA)
These methods can help to determine the relationship between the variables in the experiment and the significance of the results.
Discussion of Results
The observed trends in the reaction rates can be explained by several factors, including the concentration of the reactants, the temperature, and the presence of a catalyst.
The concentration of the reactants is a major factor that affects the reaction rate. The higher the concentration of the reactants, the more likely they are to collide with each other and react. This is because the probability of a collision between two molecules is proportional to the square of the concentration of each molecule.
Effect of Temperature, Energy worksheet #1 reaction rates
Temperature also affects the reaction rate. The higher the temperature, the faster the reaction rate. This is because the higher the temperature, the more energy the molecules have, and the more likely they are to have enough energy to overcome the activation energy barrier and react.
Effect of Catalyst
A catalyst is a substance that increases the reaction rate without being consumed in the reaction. Catalysts work by providing an alternative pathway for the reaction to occur, which has a lower activation energy than the uncatalyzed reaction. This means that the reaction can occur more easily and quickly in the presence of a catalyst.
Conclusion: Energy Worksheet #1 Reaction Rates
The results of this experiment provide valuable insights into the factors that influence reaction rates. The observed trends and relationships have important implications for understanding the behavior of chemical reactions and designing experiments to control and optimize reaction rates.
Implications for Understanding Reaction Rates
The findings of this experiment confirm the well-established principles of reaction kinetics. The observed increase in reaction rate with increasing concentration, temperature, and surface area highlights the importance of these factors in determining the rate of a reaction. These results reinforce the understanding that reactions proceed faster when reactants are more concentrated, have higher kinetic energy, and have greater contact with each other.
Common Queries
What is the significance of understanding reaction rates?
Understanding reaction rates is crucial in various fields, including chemical engineering, environmental science, and medicine. It allows scientists to optimize industrial processes, predict the behavior of chemical systems, and design drugs with desired reaction rates.
How can I calculate the reaction rate from experimental data?
The reaction rate can be calculated using the formula: Rate = (Change in concentration) / (Change in time). By measuring the change in concentration of reactants or products over time, you can determine the reaction rate.
What are some factors that affect reaction rates?
Reaction rates are influenced by several factors, including temperature, concentration of reactants, surface area, and the presence of catalysts.