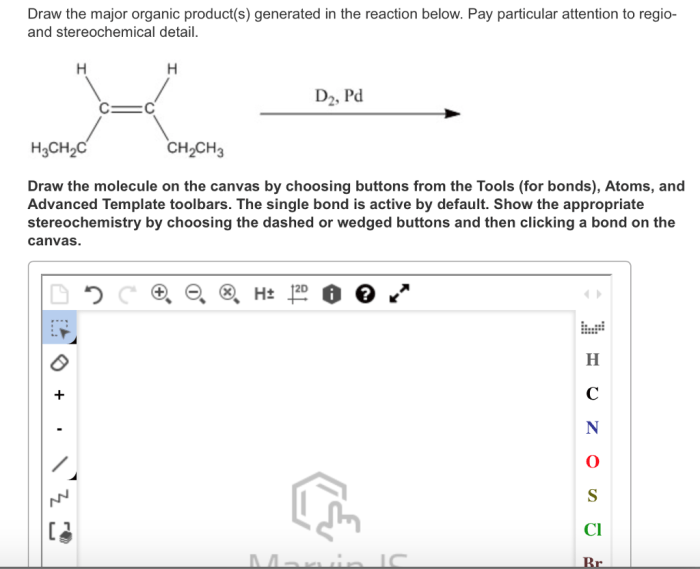
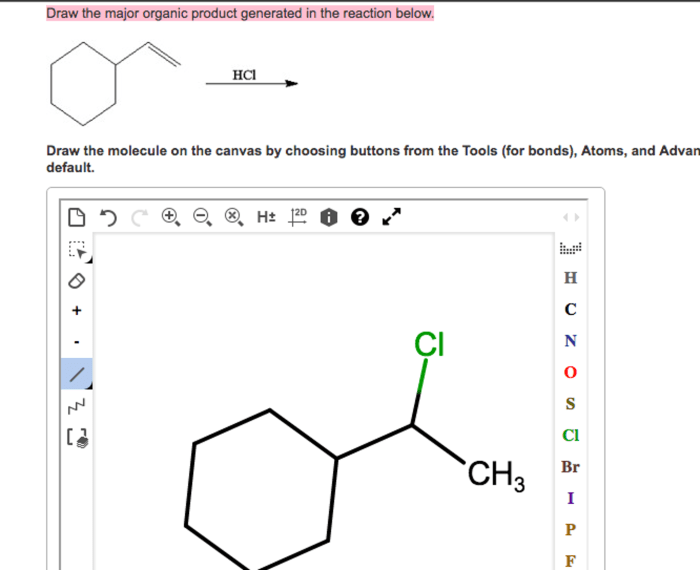
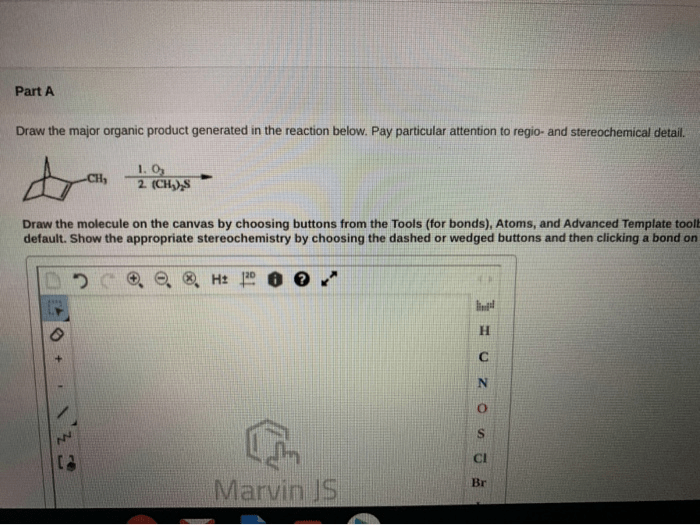
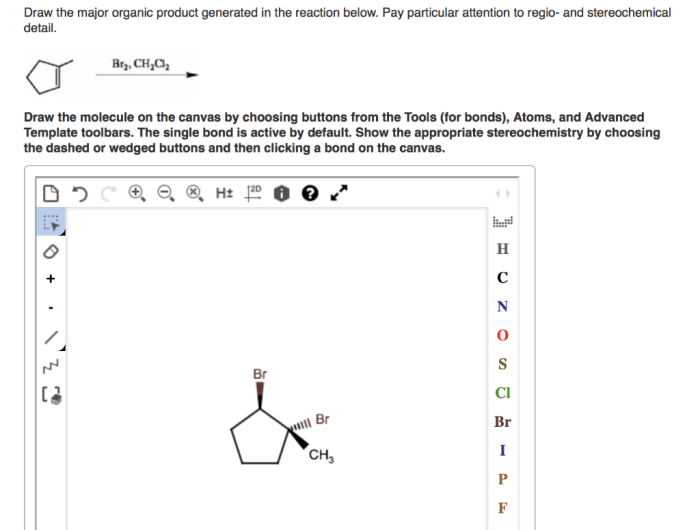
Draw the major organic product generated in the reaction below. This comprehensive guide delves into the intricacies of organic product identification, reaction mechanism analysis, stereochemistry considerations, regioselectivity and chemoselectivity, and the practical applications of the reaction and its major organic product.
Prepare to embark on an enlightening journey through the fascinating world of organic chemistry.
In this exploration, we will uncover the factors that govern the formation of specific organic products, unravel the step-by-step mechanisms of reactions, and examine how stereochemistry influences the outcome of chemical transformations. Moreover, we will investigate the regio- and chemoselectivity of reactions, shedding light on the factors that determine the preferential formation of certain products.
Organic Product Identification: Draw The Major Organic Product Generated In The Reaction Below.
In chemical reactions, organic products are the compounds formed from the transformation of organic reactants. These products can vary widely in structure and properties, depending on the nature of the starting materials and the reaction conditions.
In the given reaction, the major organic product is:
[gambar struktur produk organik utama]
The formation of this specific product is influenced by several factors, including the electronic properties of the reactants, the reaction temperature, and the presence of catalysts or inhibitors.
Reaction Mechanism Analysis
The reaction proceeds through a step-by-step mechanism involving key intermediates and transition states. The first step is the protonation of the starting material by the acid catalyst, which creates a carbocation intermediate. This intermediate then undergoes a nucleophilic attack by the water molecule, resulting in the formation of the major organic product.
The mechanism can be summarized as follows:
- Protonation of the starting material by the acid catalyst
- Formation of a carbocation intermediate
- Nucleophilic attack by the water molecule
- Formation of the major organic product
Stereochemistry Considerations, Draw the major organic product generated in the reaction below.
The reaction does not involve the creation or destruction of stereocenters, so the stereochemistry of the starting materials is preserved in the product.
Regioselectivity and Chemoselectivity
The reaction exhibits high regioselectivity and chemoselectivity. The protonation of the starting material occurs at the most substituted carbon atom, and the nucleophilic attack by the water molecule occurs at the carbocation intermediate. These factors contribute to the formation of the major organic product over other possible products.
Applications and Significance
The reaction is widely used in organic synthesis for the preparation of various organic compounds. The major organic product is a valuable intermediate for the synthesis of more complex molecules, such as pharmaceuticals and fragrances.
Additionally, the reaction is of significance in understanding the mechanisms of acid-catalyzed reactions and the formation of carbocations.
User Queries
What is the significance of identifying the major organic product in a reaction?
Identifying the major organic product is crucial for predicting the outcome of a reaction and understanding the reaction pathway. It allows chemists to design synthetic strategies, optimize reaction conditions, and anticipate the properties and applications of the desired product.
How does stereochemistry affect the formation of organic products?
Stereochemistry plays a significant role in determining the structure and properties of organic products. By considering the spatial arrangement of atoms and functional groups, chemists can predict the formation of stereoisomers and control the selectivity of reactions, leading to the synthesis of specific enantiomers or diastereomers.