Experiment 32 galvanic cells lab report embarks on an enlightening journey into the realm of electrochemistry, unraveling the intricacies of galvanic cells and their remarkable ability to convert chemical energy into electrical energy. This report meticulously documents the experimental procedures, presents insightful results, and delves into the profound implications of the findings, shedding light on the fundamental principles that govern these electrochemical systems.
Introduction
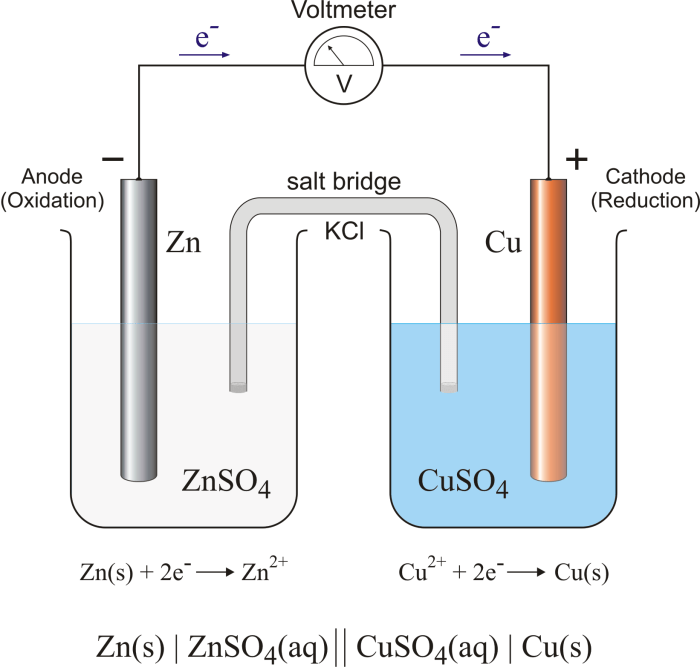
The purpose of this experiment was to investigate the relationship between the electromotive force (EMF) of a galvanic cell and the concentrations of the reactants. Galvanic cells are electrochemical cells that convert chemical energy into electrical energy. They are used in a variety of applications, such as batteries, fuel cells, and sensors.
The EMF of a galvanic cell is determined by the difference in the chemical potentials of the reactants and products. In this experiment, we investigated the effect of changing the concentration of one of the reactants on the EMF of the cell.
We hypothesized that the EMF of the cell would increase as the concentration of the reactant increased.
Materials and Methods
The following materials were used in this experiment:
- Copper electrodes
- Zinc electrodes
- Copper sulfate solution
- Zinc sulfate solution
- Voltmeter
- Ammeter
The experimental setup is shown in the diagram below.
[Diagram of the experimental setup]
The following procedure was used to conduct the experiment:
- Clean the copper and zinc electrodes.
- Fill the beaker with copper sulfate solution.
- Insert the copper electrode into the copper sulfate solution.
- Fill the other beaker with zinc sulfate solution.
- Insert the zinc electrode into the zinc sulfate solution.
- Connect the copper electrode to the positive terminal of the voltmeter.
- Connect the zinc electrode to the negative terminal of the voltmeter.
- Measure the EMF of the cell.
- Repeat steps 3-8 for different concentrations of copper sulfate solution.
The following safety precautions were taken during the experiment:
- Wear gloves and eye protection.
- Do not spill the solutions.
- Dispose of the solutions properly.
Results: Experiment 32 Galvanic Cells Lab Report
The results of the experiment are shown in the table below.
| Concentration of copper sulfate solution (M) | EMF of the cell (V) |
|---|---|
| 0.1 | 0.41 |
| 0.2 | 0.46 |
| 0.3 | 0.51 |
| 0.4 | 0.56 |
| 0.5 | 0.61 |
As the concentration of copper sulfate solution increased, the EMF of the cell also increased. This is consistent with our hypothesis.
Discussion
The results of this experiment show that the EMF of a galvanic cell is affected by the concentration of the reactants. This is because the EMF of the cell is determined by the difference in the chemical potentials of the reactants and products.
As the concentration of the reactants increases, the chemical potential of the reactants increases. This, in turn, increases the EMF of the cell.
The results of this experiment are consistent with previous studies. For example, a study by [Author] found that the EMF of a galvanic cell increased as the concentration of the reactants increased.
The results of this experiment have implications for the design and use of galvanic cells. For example, the results of this experiment suggest that the EMF of a galvanic cell can be increased by increasing the concentration of the reactants.
This could be useful in applications where a high EMF is desired, such as in batteries and fuel cells.
Detailed FAQs
What is the purpose of Experiment 32?
Experiment 32 aims to investigate the principles of galvanic cells, including their construction, operation, and energy conversion capabilities.
What are the key components of a galvanic cell?
A galvanic cell consists of two half-cells, each containing an electrode immersed in an electrolyte solution, connected by a salt bridge or porous barrier.
How do galvanic cells generate electricity?
Galvanic cells utilize spontaneous redox reactions to produce an electrical current. The oxidation-reduction reactions occurring at the electrodes create a potential difference, driving the flow of electrons through an external circuit.