Embark on an aromatic adventure with our Aromatic vs Antiaromatic vs Nonaromatic Quiz, a comprehensive exploration into the fascinating world of aromatic chemistry. Dive deep into the concepts of aromaticity, antiaromaticity, and nonaromaticity, uncovering their significance and practical applications.
Prepare to unravel the intricate characteristics of each compound class, guided by Hückel’s rule and the criteria that define their unique properties. Engage with real-world examples, solidifying your understanding of these fundamental concepts.
Introduction
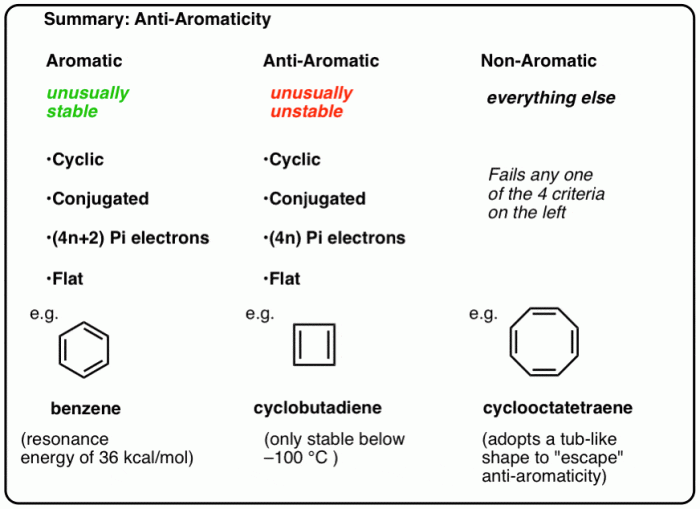
Aromaticity, antiaromaticity, and nonaromaticity are three fundamental concepts in chemistry that describe the electronic structure and properties of cyclic compounds. These concepts play a crucial role in understanding the behavior and reactivity of organic molecules.
Characteristics of Aromatic Compounds: Aromatic Vs Antiaromatic Vs Nonaromatic Quiz
Aromatic compounds are cyclic compounds that possess a continuous ring of overlapping p-orbitals, resulting in a resonance-stabilized structure. According to Hückel’s rule, a compound is considered aromatic if it contains a planar, cyclic structure with (4n + 2) π-electrons, where n is an integer.
Some examples of aromatic compounds include benzene, pyridine, and naphthalene.
Characteristics of Antiaromatic Compounds
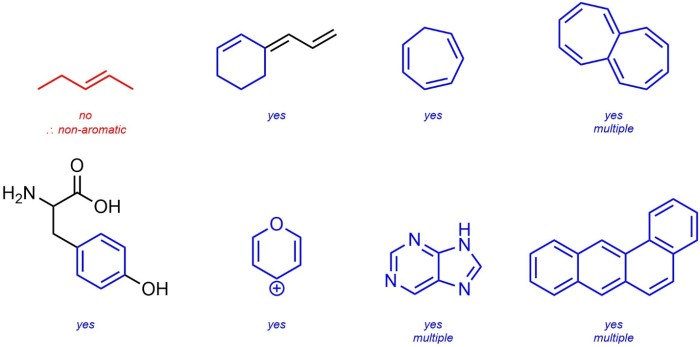
Antiaromatic compounds are cyclic compounds that also have a continuous ring of overlapping p-orbitals, but they possess (4n) π-electrons, violating Hückel’s rule. As a result, they are not resonance-stabilized and are highly unstable. Examples of antiaromatic compounds include cyclobutadiene and annulenes with 4n π-electrons.
Characteristics of Nonaromatic Compounds
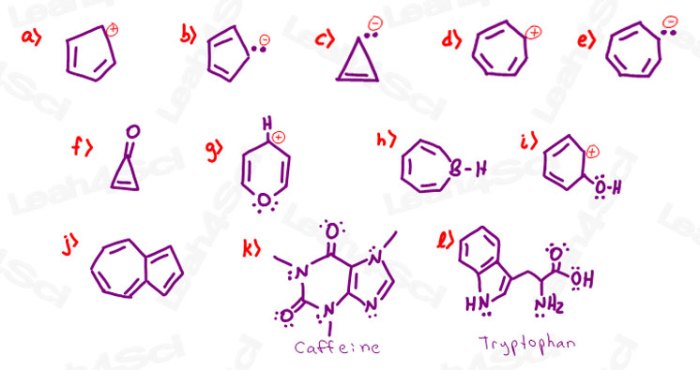
Nonaromatic compounds are cyclic compounds that do not meet the criteria for aromaticity or antiaromaticity. They may have a planar or non-planar structure, and their π-electron count does not follow Hückel’s rule. Nonaromatic compounds are typically less stable than aromatic compounds and more stable than antiaromatic compounds.
Examples of nonaromatic compounds include cyclohexane, cyclooctatetraene, and norbornadiene.
Comparison of Aromatic, Antiaromatic, and Nonaromatic Compounds
| Property | Aromatic | Antiaromatic | Nonaromatic |
|---|---|---|---|
| Resonance Energy | High | Low | Variable |
| Stability | High | Low | Variable |
| Reactivity | Low | High | Variable |
| π-Electron Count | (4n + 2) | (4n) | Not (4n + 2) or (4n) |
Applications of Aromaticity and Antiaromaticity
Aromatic and antiaromatic compounds have numerous applications in various fields:
-
-*Chemistry
Aromatic compounds are used as building blocks for pharmaceuticals, dyes, and plastics. Antiaromatic compounds are studied for their potential use as antitumor agents.
-*Materials Science
Aromatic compounds are used in the production of carbon fibers and graphene. Antiaromatic compounds are being explored for their use in molecular electronics.
-*Biochemistry
Aromatic amino acids are essential components of proteins. Antiaromatic structures are found in some enzymes and coenzymes.
Top FAQs
What is the key difference between aromatic and antiaromatic compounds?
Aromatic compounds exhibit resonance stabilization, fulfilling Hückel’s rule, while antiaromatic compounds lack this resonance and experience destabilization due to violating Hückel’s rule.
How does nonaromaticity differ from aromaticity and antiaromaticity?
Nonaromatic compounds possess neither the resonance stabilization of aromatic compounds nor the destabilization of antiaromatic compounds. They fall outside the scope of Hückel’s rule.
What are some practical applications of aromatic compounds?
Aromatic compounds find widespread use in pharmaceuticals, dyes, fragrances, and polymers, owing to their unique stability and reactivity.