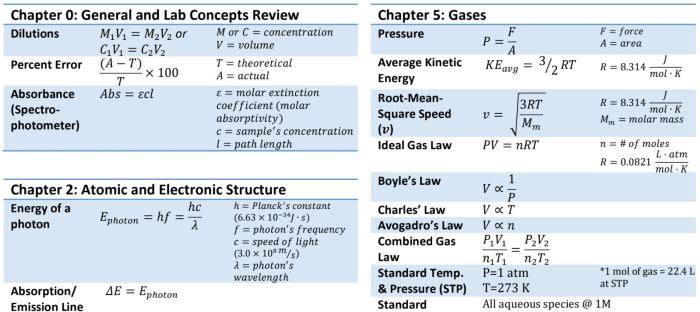
Chapter 6 review chemical bonding – Chapter 6 Review: Chemical Bonding embarks on an enthralling journey into the intricate world of chemical bonding, where the fundamental forces that hold atoms together shape the properties of matter. This chapter delves into the diverse types of bonds, exploring their characteristics, influences, and applications, providing a comprehensive understanding of the molecular interactions that govern our universe.
Delving deeper into the realm of chemical bonding, we uncover the factors that orchestrate bond formation, examining the interplay of electronegativity, atomic radius, and bond order. These factors dance together, influencing bond strength and stability, shaping the molecular landscapes we observe.
Types of Chemical Bonding: Chapter 6 Review Chemical Bonding
Chemical bonding is the force that holds atoms together to form molecules and compounds. There are several types of chemical bonds, each with its own unique characteristics and properties.
Ionic Bonding
Ionic bonding occurs when one atom transfers one or more electrons to another atom, creating two oppositely charged ions. The positive ion is called a cation, and the negative ion is called an anion. Ionic bonds are typically formed between a metal and a non-metal.Examples
of ionic compounds include sodium chloride (NaCl), potassium chloride (KCl), and calcium oxide (CaO).Ionic bonds are strong and result in the formation of crystalline solids. Ionic compounds are typically soluble in water and have high melting and boiling points.
Covalent Bonding
Covalent bonding occurs when two atoms share one or more pairs of electrons. The shared electrons are attracted to the nuclei of both atoms, creating a strong bond. Covalent bonds are typically formed between two non-metals.Examples of covalent compounds include hydrogen gas (H2), water (H2O), and methane (CH4).Covalent
bonds are generally weaker than ionic bonds and result in the formation of molecular solids, liquids, or gases. Covalent compounds are typically insoluble in water and have lower melting and boiling points than ionic compounds.
Metallic Bonding
Metallic bonding occurs between metal atoms. In metallic bonding, the metal atoms share their valence electrons in a sea of electrons. The valence electrons are not attached to any particular atom but are free to move throughout the metal.Metallic bonds are very strong and result in the formation of shiny, malleable, and ductile solids.
Metals are good conductors of heat and electricity.Examples of metals include iron, copper, and aluminum.
Hydrogen Bonding
Hydrogen bonding is a special type of dipole-dipole interaction that occurs between a hydrogen atom bonded to a highly electronegative atom (such as oxygen, nitrogen, or fluorine) and another electronegative atom. The hydrogen atom has a partial positive charge, and the electronegative atom has a partial negative charge.
The partial positive charge of the hydrogen atom is attracted to the partial negative charge of the electronegative atom, creating a hydrogen bond.Hydrogen bonds are weaker than ionic or covalent bonds, but they can still have a significant impact on the properties of a substance.
Hydrogen bonds are responsible for the high boiling point of water and the formation of many biological molecules, such as DNA and proteins.Examples of substances that exhibit hydrogen bonding include water, alcohols, and carboxylic acids.
Factors Affecting Bond Formation
The formation of chemical bonds is influenced by several key factors, including electronegativity, atomic radius, and bond order. These factors determine the strength and stability of the resulting bond.
Electronegativity
Electronegativity measures the attraction of an atom for electrons. Atoms with high electronegativity tend to attract electrons towards themselves, while those with low electronegativity are more willing to give up electrons. In a chemical bond, the more electronegative atom will have a greater share of the electron density, resulting in a stronger bond.
Atomic Radius
Atomic radius refers to the size of an atom. Smaller atoms have a higher electron density, making them more likely to form strong bonds. Larger atoms, with their lower electron density, tend to form weaker bonds.
Bond Order
Bond order describes the number of electron pairs shared between two atoms. Single bonds, with one shared pair, are weaker than double bonds, with two shared pairs, and triple bonds, with three shared pairs. The higher the bond order, the stronger the bond.
Molecular Geometry and Bonding
Molecular geometry refers to the three-dimensional arrangement of atoms within a molecule. It is closely related to the type of chemical bonding present in the molecule.
VSEPR Theory
The Valence Shell Electron Pair Repulsion (VSEPR) theory is a model used to predict the molecular geometry of a molecule based on the number of valence electron pairs around the central atom. The theory states that electron pairs repel each other and will arrange themselves in a way that minimizes repulsion.
- Linear:2 electron pairs around the central atom (bond angle: 180°)
- Trigonal Planar:3 electron pairs around the central atom (bond angle: 120°)
- Tetrahedral:4 electron pairs around the central atom (bond angle: 109.5°)
- Trigonal Pyramidal:3 electron pairs and 1 lone pair around the central atom (bond angle:<109.5°)
- Bent:2 electron pairs and 2 lone pairs around the central atom (bond angle:<120°)
Bonding in Solids, Liquids, and Gases
In solids, liquids, and gases, the type of bonding that holds atoms or molecules together affects the physical properties of the substance.
Solids
In solids, atoms are held together by strong covalent or ionic bonds, resulting in a rigid structure. This strong bonding gives solids their characteristic properties, such as definite shape, volume, and high density. Examples of solids include metals, ceramics, and crystals.
Liquids
In liquids, molecules are held together by weaker intermolecular forces, such as van der Waals forces or hydrogen bonds. These forces are not as strong as the bonds in solids, allowing molecules to move past each other. Liquids have a definite volume but no definite shape, and they are less dense than solids.
Examples of liquids include water, oil, and alcohol.
Gases
In gases, molecules are held together by very weak intermolecular forces. The molecules are far apart and have high kinetic energy, allowing them to move freely in all directions. Gases have no definite shape or volume and are the least dense of the three states of matter.
Examples of gases include air, helium, and hydrogen.
Applications of Chemical Bonding
Chemical bonding is not just a theoretical concept but has far-reaching applications in various fields, shaping our technological advancements. Understanding the principles of chemical bonding allows scientists and engineers to design and create materials with specific properties, leading to groundbreaking innovations.
Materials Science
Chemical bonding plays a crucial role in materials science, enabling the development of new materials with tailored properties. By understanding the types and strengths of chemical bonds, scientists can design materials with specific characteristics, such as strength, durability, and conductivity.
This knowledge has led to the creation of advanced materials like carbon nanotubes, graphene, and shape-memory alloys, which are revolutionizing industries from construction to electronics.
Pharmaceuticals, Chapter 6 review chemical bonding
In the field of pharmaceuticals, chemical bonding is essential for understanding the interactions between drugs and biological systems. By studying the chemical bonds involved in drug-receptor interactions, researchers can design more effective and targeted therapies. This has led to the development of new drugs with improved efficacy and reduced side effects, transforming the treatment of various diseases.
Energy Storage
Chemical bonding is also central to energy storage technologies, such as batteries and fuel cells. The ability to control and manipulate chemical bonds allows scientists to develop more efficient and durable energy storage systems. For example, the development of lithium-ion batteries, which rely on the intercalation and de-intercalation of lithium ions, has revolutionized portable electronics and electric vehicles.
Questions Often Asked
What is the difference between ionic and covalent bonds?
Ionic bonds involve the transfer of electrons, while covalent bonds involve the sharing of electrons.
How does electronegativity affect bond strength?
Electronegativity measures the attraction of an atom for electrons. The greater the difference in electronegativity between two atoms, the stronger the bond.
What is the VSEPR theory?
The VSEPR theory predicts the molecular geometry based on the number of electron pairs around the central atom.